The Gut Microbiome Alterations is related to Autism Spectrum Disorders
Autism spectrum disorder (ASD) is a hereditary linked disorder of brain function with symptoms, such as restricted forms of behaviors and activities, or persistent discrepancies in societal interaction and communication. ASD has a significant impact on the growth and development of children and on the general public. The assessed incidence of ASD in 2012 was about 14.6 per 1,000 children (aged 8 years), and its occurrence was relatively higher in boys (23.6 per 1,000) when compared to girls (5.3 per 1,000). As per the estimate, in the United States alone, the cost of caring for a child with ASD was about $1.4 million, while in the United Kingdom, it was reported to be £0.92 million (Li et al. 2017). The main costs associated with the care of children with ASD are special education services and a loss of parental productivity. Thus, the financial effects of ASD have driven scientists to hunt for effective medications (Buescher et al. 2014; Li et al. 2017).
ASD is described by numerous clinical endophenotypes, and is hypothetically connected with certain comorbidities. As per the current recommendations, gastrointestinal symptoms are the reasons for the comorbidity in patients with ASD, however underlying mechanisms are unfamiliar. Several investigations have revealed that patients with ASD have alterations in the fecal flora composition, and the gut microbiome metabolic products. The dietary constituents along with metabolic activities of the microbiome are now assumed to be linked to alterations in behavior and cognition, mainly in patients having neurodegenerative diseases. The gut microbiome have a significant role in the development of the brain and controls the behavior via the neuroimmune, neuroendocrine, and autonomic nervous systems. There is a bidirectional communication between the central nervous system and the gastrointestinal tract (brain-gut axis) and the gut microbiome functions. As stated by most recent studies, collective pathogenetic elements and pathophysiological mechanisms probably associating to gastrointestinal disturbances and ASD include intestinal inflammation with or without autoimmunity, gluten-related disorders (celiac disease, wheat allergy, non-celiac gluten sensitivity), immunoglobulin E-mediated and/or cell-mediated gastrointestinal food allergies, abdominal pain, dysautonomia connected with gastroesophageal reflux and gastrointestinal dysmotility. In addition, dysregulation of the gut microbiome has the capability to upset intestinal permeability, motility and sensitivity, and mucosal immune function.
The human gut contains about 1000 grams of bacteria, and approximately about 9.9 million numbers of bacterial genes exist in the gut i.e., the ratio of microbiome DNA and host DNA is 10:1. Gastrointestinal indications are noticeable in ASD patients. Researchers have observed more gastrointestinal syndromes, including diarrhea and constipation in children having ASD compared with their unaffected siblings. ASD patients with gastrointestinal syndromes display substantial interactive or behavioral manifestations, including self-injury, aggression and anxiety. Many studies have demonstrated that the gut microbiota composition and function is directly or indirectly linked with ASD signs, partly by prompting the immune system and metabolic rate. Patients with ASD are observed to have a higher percentage of abnormal intestinal permeability leading to greater antigenic load from the gastrointestinal tract. ASD-associated cytokines, such as interferon-γ (IFN-γ), interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) in addition to lymphocytes present in the circulation cross the blood-brain barrier (BBB). Successively, IL-6, IL-1β and TNF-α bind to brain endothelial cells and encourage immune responses in the brain. Variations in the gut microbiota composition and their metabolic products secretion are generally noticed in ASD patients and in animal models of ASD. In a mouse model study featuring ASD, researchers have found that gastrointestinal barrier defects caused microbiota alterations. They observed more abundant bacteria, belonging to Prevotellaceae, Porphyromonadaceae, unclassified Bacteroidales and Lachnospiraceae in offspring of mothers with maternal immune activation, while in control offspring, Erysipelotrichaceae, Ruminococcaceae and Alcaligenaceae were found to occur more abundantly. The gut microbiota diversity in children with ASD is observed to be very less as compared to that of children without ASD. In ASD patients, higher levels of Desulfovibrio, Lactobacillus, Bacteroidetes, Clostridium, Caloramator and Sarcina, and lower levels of Bifidobacterium and Firmicutes are observed. Further, children with ASD and gastrointestinal syndromes possess inferior loads of unclassified Veillonellaceae and the genera, Coprococcus, and Prevotella than that found in gastrointestinal syndrome-free neurotypical children. Clostridium histolyticum, found in the fecal samples of children with ASD was at a higher level when compared with healthy children without ASD. Moreover, altered levels of Prevotella, Bifidobacterium, and Sutterella are found in children with ASD. Researchers also have observed that Candida species (Candida krusei and C. glabrata) were 2-times higher in individuals affected with autism than in normal individuals. Also, it is observed that Candida species can release ammonia and toxins that can encourage autistic behaviors.
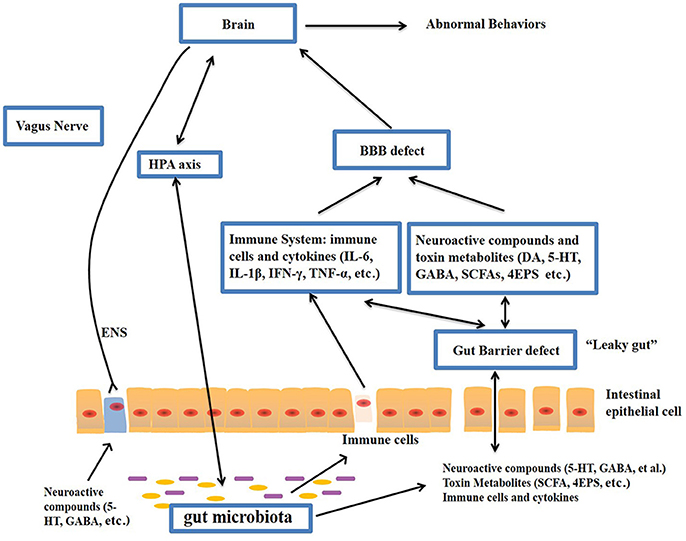
The gut–brain axis is viewed as a way of interaction between the gut microbiome and the brain, and it is a two-way communication structure. The outcome of several studies suggests that the gut-brain axis contributes to the origin and development of ASD. In a comprehensive review by Li et al. (2017) stated that the gut flora affects functions of the brain via the neuroendocrine, neuroimmune and autonomic nervous systems and through toxin production by microbiota (Figure 1). The presence of millions of neurons in the mucosa of the gastrointestinal tract constitutes the enteric nervous system and modulate various gastrointestinal functions. Hence, the gut is recognized as ‘the second brain’. ?The potential interactions between the gut microbiota and the pathogenesis of ASD is outlined in Figure 1.
Figure-1: Potential relationships between the microbiota and ASD (the gut-brain axis). The production of metabolites, such as SCFAs and toxin metabolites, by certain microbiota (e.g., Lactobacillus) can cross the “leaky gut” to affect brain function. Some microbiota can produce neuroactive compounds (e.g., 5-HT and GABA) that cross the “leaky gut” and influence brain function and induce abnormal behaviors. These neuroactive compounds can directly influence the HPA axis and increase circulating levels of cortisol. Metabolites, certain microbiota and neuroactive compounds can activate enteric neurons and affect brain function through the vagus nerve. Some microbiota and metabolites can activate gut immune cells, which can release cytokines into circulation. 4-EPS, 4-ethylphenyl sulfate; 5-HT, serotonin; HPA, hypothalamic–pituitary–adrenal; SCFAs, short-chain fatty acids; BBB, blood-brain barrier; 5-HT, 5-hydroxytryptamine; ENS, enteric nervous system; GABA, γ-aminobutyric acid; DA, dopamine. (Adapted from Li et al. (2017); doi: 10.3389/fncel.2017.00120)
The gut microbiota secretes certain metabolites, such as phenolics, short-chain fatty acids (acetic acid, proprionic acid, isobutyric acid, butyrate, valeric acid and isovaleric acid), and free amino acids that affect ASD-like behaviors via the vagal pathways mediating the microbiome-brain-gut axis communication. Likewise, the gut microbiota releases neuroactive compounds (dopamine, 5-HT, γ-aminobutyric acid, and histamine), which trigger or impede central neurons via the vagus nerve.
Currently, there are no available effective therapies for ASD. Parents often take their children to obtain mediations that are personalized to their specific needs. Recent evidences have indicated that the gut microbiota modulation can be a potential therapy in children having ASD. In this regard, prebiotics, probiotics, fecal microbiota transplantation and a suitable personalized diet have gained substantial attention in recent days. Moreover, microbiome-facilitated therapies are relatively safe and effective. Recent clinical studies have reported that ASD patient’s symptoms can be improved by treatments that normalize the gut microbiota. Nevertheless, more research studies involving more participants are required to know about the treatments that supports the gut microbiome in overcoming ASD symptoms.
Keywords:
The gut microbiota, microbiome, Autism spectrum disorder, Brain-gut axis, Probiotics, Fecal microbiota transplantation, Microbiome-mediated therapies
References:
- Li Q, Han Y, Dy AB, Hagerman RJ. The gut microbiota and autism spectrum disorders. Frontiers in cellular neuroscience. 2017 Apr 28;11:120.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015 Jan 1;138:179-87.
- Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA pediatrics. 2014 Aug 1;168(8):721-8.
Autism spectrum disorder (ASD) is a hereditary linked disorder of brain function with symptoms, such as restricted forms of behaviors and activities, or persistent discrepancies in societal interaction and communication. ASD has a significant impact on the growth and development of children and on the general public. The assessed incidence of ASD in 2012 was about 14.6 per 1,000 children (aged 8 years), and its occurrence was relatively higher in boys (23.6 per 1,000) when compared to girls (5.3 per 1,000). As per the estimate, in the United States alone, the cost of caring for a child with ASD was about $1.4 million, while in the United Kingdom, it was reported to be £0.92 million (Li et al. 2017). The main costs associated with the care of children with ASD are special education services and a loss of parental productivity. Thus, the financial effects of ASD have driven scientists to hunt for effective medications (Buescher et al. 2014; Li et al. 2017).
ASD is described by numerous clinical endophenotypes, and is hypothetically connected with certain comorbidities. As per the current recommendations, gastrointestinal symptoms are the reasons for the comorbidity in patients with ASD, however underlying mechanisms are unfamiliar. Several investigations have revealed that patients with ASD have alterations in the fecal flora composition, and the gut microbiome metabolic products. The dietary constituents along with metabolic activities of the microbiome are now assumed to be linked to alterations in behavior and cognition, mainly in patients having neurodegenerative diseases. The gut microbiome have a significant role in the development of the brain and controls the behavior via the neuroimmune, neuroendocrine, and autonomic nervous systems. There is a bidirectional communication between the central nervous system and the gastrointestinal tract (brain-gut axis) and the gut microbiome functions. As stated by most recent studies, collective pathogenetic elements and pathophysiological mechanisms probably associating to gastrointestinal disturbances and ASD include intestinal inflammation with or without autoimmunity, gluten-related disorders (celiac disease, wheat allergy, non-celiac gluten sensitivity), immunoglobulin E-mediated and/or cell-mediated gastrointestinal food allergies, abdominal pain, dysautonomia connected with gastroesophageal reflux and gastrointestinal dysmotility. In addition, dysregulation of the gut microbiome has the capability to upset intestinal permeability, motility and sensitivity, and mucosal immune function.
The human gut contains about 1000 grams of bacteria, and approximately about 9.9 million numbers of bacterial genes exist in the gut i.e., the ratio of microbiome DNA and host DNA is 10:1. Gastrointestinal indications are noticeable in ASD patients. Researchers have observed more gastrointestinal syndromes, including diarrhea and constipation in children having ASD compared with their unaffected siblings. ASD patients with gastrointestinal syndromes display substantial interactive or behavioral manifestations, including self-injury, aggression and anxiety. Many studies have demonstrated that the gut microbiota composition and function is directly or indirectly linked with ASD signs, partly by prompting the immune system and metabolic rate. Patients with ASD are observed to have a higher percentage of abnormal intestinal permeability leading to greater antigenic load from the gastrointestinal tract. ASD-associated cytokines, such as interferon-γ (IFN-γ), interleukin-6 (IL-6), IL-1β and tumor necrosis factor-α (TNF-α) in addition to lymphocytes present in the circulation cross the blood-brain barrier (BBB). Successively, IL-6, IL-1β and TNF-α bind to brain endothelial cells and encourage immune responses in the brain. Variations in the gut microbiota composition and their metabolic products secretion are generally noticed in ASD patients and in animal models of ASD. In a mouse model study featuring ASD, researchers have found that gastrointestinal barrier defects caused microbiota alterations. They observed more abundant bacteria, belonging to Prevotellaceae, Porphyromonadaceae, unclassified Bacteroidales and Lachnospiraceae in offspring of mothers with maternal immune activation, while in control offspring, Erysipelotrichaceae, Ruminococcaceae and Alcaligenaceae were found to occur more abundantly. The gut microbiota diversity in children with ASD is observed to be very less as compared to that of children without ASD. In ASD patients, higher levels of Desulfovibrio, Lactobacillus, Bacteroidetes, Clostridium, Caloramator and Sarcina, and lower levels of Bifidobacterium and Firmicutes are observed. Further, children with ASD and gastrointestinal syndromes possess inferior loads of unclassified Veillonellaceae and the genera, Coprococcus, and Prevotella than that found in gastrointestinal syndrome-free neurotypical children. Clostridium histolyticum, found in the fecal samples of children with ASD was at a higher level when compared with healthy children without ASD. Moreover, altered levels of Prevotella, Bifidobacterium, and Sutterella are found in children with ASD. Researchers also have observed that Candida species (Candida krusei and C. glabrata) were 2-times higher in individuals affected with autism than in normal individuals. Also, it is observed that Candida species can release ammonia and toxins that can encourage autistic behaviors.
The gut–brain axis is viewed as a way of interaction between the gut microbiome and the brain, and it is a two-way communication structure. The outcome of several studies suggests that the gut-brain axis contributes to the origin and development of ASD. In a comprehensive review by Li et al. (2017) stated that the gut flora affects functions of the brain via the neuroendocrine, neuroimmune and autonomic nervous systems and through toxin production by microbiota (Figure 1). The presence of millions of neurons in the mucosa of the gastrointestinal tract constitutes the enteric nervous system and modulate various gastrointestinal functions. Hence, the gut is recognized as ‘the second brain’. ?The potential interactions between the gut microbiota and the pathogenesis of ASD is outlined in Figure 1.
Figure-1: Potential relationships between the microbiota and ASD (the gut-brain axis). The production of metabolites, such as SCFAs and toxin metabolites, by certain microbiota (e.g., Lactobacillus) can cross the “leaky gut” to affect brain function. Some microbiota can produce neuroactive compounds (e.g., 5-HT and GABA) that cross the “leaky gut” and influence brain function and induce abnormal behaviors. These neuroactive compounds can directly influence the HPA axis and increase circulating levels of cortisol. Metabolites, certain microbiota and neuroactive compounds can activate enteric neurons and affect brain function through the vagus nerve. Some microbiota and metabolites can activate gut immune cells, which can release cytokines into circulation. 4-EPS, 4-ethylphenyl sulfate; 5-HT, serotonin; HPA, hypothalamic–pituitary–adrenal; SCFAs, short-chain fatty acids; BBB, blood-brain barrier; 5-HT, 5-hydroxytryptamine; ENS, enteric nervous system; GABA, γ-aminobutyric acid; DA, dopamine. (Adapted from Li et al. (2017); doi: 10.3389/fncel.2017.00120)
The gut microbiota secretes certain metabolites, such as phenolics, short-chain fatty acids (acetic acid, proprionic acid, isobutyric acid, butyrate, valeric acid and isovaleric acid), and free amino acids that affect ASD-like behaviors via the vagal pathways mediating the microbiome-brain-gut axis communication. Likewise, the gut microbiota releases neuroactive compounds (dopamine, 5-HT, γ-aminobutyric acid, and histamine), which trigger or impede central neurons via the vagus nerve.
Currently, there are no available effective therapies for ASD. Parents often take their children to obtain mediations that are personalized to their specific needs. Recent evidences have indicated that the gut microbiota modulation can be a potential therapy in children having ASD. In this regard, prebiotics, probiotics, fecal microbiota transplantation and a suitable personalized diet have gained substantial attention in recent days. Moreover, microbiome-facilitated therapies are relatively safe and effective. Recent clinical studies have reported that ASD patient’s symptoms can be improved by treatments that normalize the gut microbiota. Nevertheless, more research studies involving more participants are required to know about the treatments that supports the gut microbiome in overcoming ASD symptoms.
Keywords:
The gut microbiota, microbiome, Autism spectrum disorder, Brain-gut axis, Probiotics, Fecal microbiota transplantation, Microbiome-mediated therapies
References:
- Li Q, Han Y, Dy AB, Hagerman RJ. The gut microbiota and autism spectrum disorders. Frontiers in cellular neuroscience. 2017 Apr 28;11:120.
- Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiology & behavior. 2015 Jan 1;138:179-87.
- Buescher AV, Cidav Z, Knapp M, Mandell DS. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA pediatrics. 2014 Aug 1;168(8):721-8.
The gut microbiome have a role in Dementia
Dementia, as a comprehensive type of brain disease is known to upset an individual’s daily activities. The disease is characterized by the deteriorated memory, thinking ability, behavior and the capability to carry out everyday functions. Globally, ~47.5 million individuals are affected and the prevalence of dementia is increasing continuously. Dementia leads to disability and dependency amongst the aged people, and thus has a colossal physical, mental, societal and economic effect on families, caregivers, and the general public. The common form of dementia is the Alzheimer’s disease, which account to about 60-70% of the cases followed by other forms, such as Lewy body dementia, vascular dementia, frontotemporal dementia, and Parkinson’s disease with dementia.
Recent research advancements have deciphered the role of gut microbiome and its composition associated with the gut permeability and inflammation in dementia patients. Also, it is hypothesized that dysbiosis (an impaired microbiota) may augment the gut permeability, microbial translocation and trigger an inflammatory-immune response, which may perhaps encourage pathogenesis and progression of dementia. In this regard, a novel approach is suggested for the management of these health disorders, and as an adjuvant for psychiatric treatment of neurological disorders, including dementia and other interrelated diseases via modulating the microbiota composition (e.g., with the use of probiotics).
The expansion of advanced high-throughput analytical techniques including next generation sequencing has disclosed the possible role of the commensal microbial population, particularly the gut microbiome in several human diseases. Lately, the theory of the gut brain-axis has been well-established. Accordingly, a communication exists between the gut and brain site to control and mediate the process of many biological pathways involving the autonomic and enteric nervous system, the neuroendocrine system and the immune system. However, any deviations in such communication due to dysbiosis may involve in disease development. For example, the enteric microbiota i.e., Bifidobacterium and Escherichia, which are considered as both commensal and pathogenic microbes are identified to have interactions between the brain and gut axis.
The gut microbiota supports numerous daily activities of the brain, comprising the regulation of the hypothalamic-pituitary-adrenal (HPA) axis activation state. The HPA axis activation releases cortisol, which in turn manage the triggering of microglia of the brain, and influence to release cytokines. This action rule the functions of the central nervous system through various communications, including vagal nerve and adrenergic nerve activation in addition to the secretion of many molecular candidates (endocrine hormones, neuropeptides, neurotransmitters, and immunomodulators). The stress hormones of hosts, for example noradrenaline might affect signalling between bacteria or their activities in the gut and lead to changes in the microbial diversity and overall functions of the gut microbiota. Nevertheless, these gut bacteria synthesize and release many neurotransmitters and neuromodulators, and induce the synthesis as well as the release of neuropeptides from enteroendocrine cells. For instance: the species of Bifidobacterium and Lactobacillus produce short-chain fatty acids; spore-forming bacteria produce 5-HT; Bacillus, Escherichia and Saccharomyces spp. secrete norepinephrine; Bacillus and Lactobacillus spp. produce dopamine and acetylcholine, respectively. In specific, recent findings have suggested that microglia maturation and function is governed by the short-chain fatty acids produced by the gut microbiota, and hence highlighted on the possible usefulness of the gut microbiome as an impending diagnostic target in dementia and its cure.
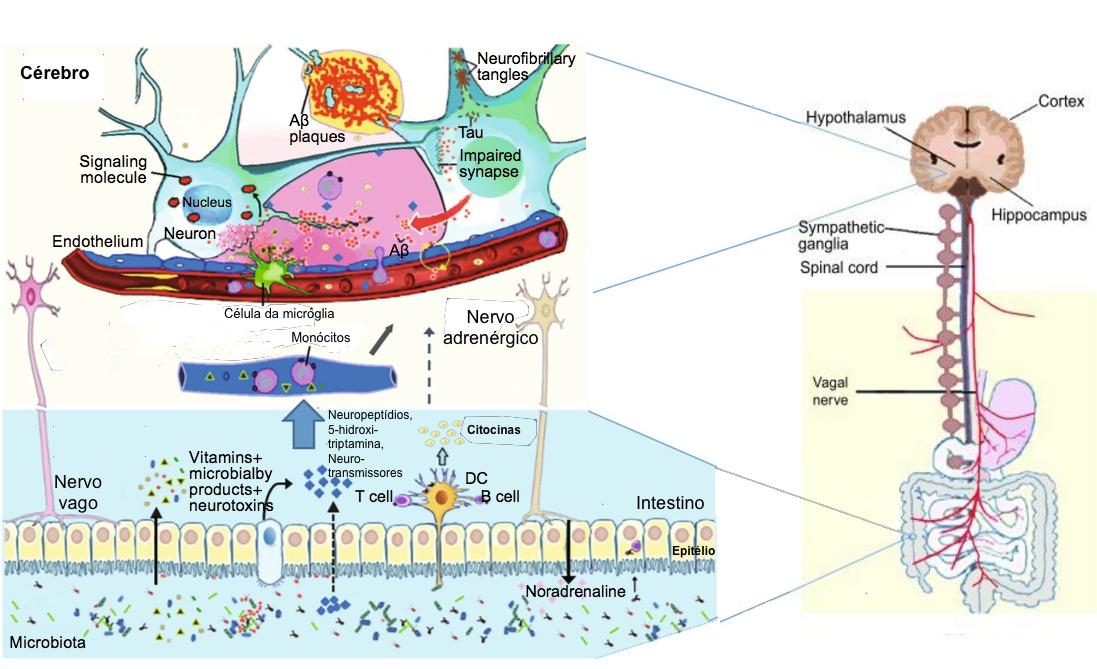
The gut microbiota regulates neuro-inflammations and the HPA axis activity, and might lead to Alzheimer’s disease. The neuropathological features of Alzheimer’s disease are the two pathologic protein aggregates, namely amyloid beta (Aβ) plaques and hyper-phosphorylated tangles of tau-protein, which cause neuro-inflammation, mainly facilitated by the innate immune system. This process mainly includes microglia cells that represent the resident macrophages of the brain. Though microglia cells can remove extracellular Aβ aggregates, in later phases of the disease, cells remain in a dystrophic state and fail to exert their positive actions. In addition, the inflammation and the pathogens (e.g., bacteria: Salmonella enterica; fungi: Candida albicans; viruses: herpes simplex virus; and parasites: Toxoplasma gondii) interactions can also lead to the development of Alzheimer’s disease. Thus, a strong gut microbiota may pay a significant role in preventing general infections by restricting pathogen growth, improving the microbial barriers, and enhancing the immune response. The representation of a few key factors in the Alzheimer’s disease pathogenesis is given in the figure-1.
Figure -1: Schematic of some key players in the pathogenesis of AD. The gut microbiota regulation of neuro-inflammation and the hypothalamic–pituitary–adrenal (HPA) axis activity and may lead to AD. The bacterial products that gain access to the brain through the bloodstream and the area postrema, via cytokine release from mucosal immune cells, through the release of gut hormones such as 5-HT from EEC cells, or via afferent neural pathways, including the vagal nerve. NP: Neuropeptide; NT: Neurotransmitter; 5-HT: 5-hydroxytryptamine; DC: Dendritic cell; EEC: Enteroendocrine cell; Aβ: amyloid beta protein; AD: Alzheimer’s disease.
(Adapted from Alkasir et al. 2017; doi: 10.1007/s13238-016-0338-6).
In the course of ageing, the gut microbiome composition undergoes fluctuations. In aged people, decreased microbial diversity with a loss of helpful taxa and increased facultative pathogens have been observed. Further, aging is connected with inflammation, which is also represented as inflammaging, which is linked with the accelerated gut permeability, mucosal inflammation and bacterial translocation. As aging is one of the chief risk factors of dementia, it is expected that the gut-brain axis is disapprovingly involved in the development of dementia. Considering this, a precise diet and the surrounding environment play a vital role in shaping the good microbiome composition and help in controlling many metabolic disorders. Some of the animal investigations have suggested that dementia is associated with fluctuations in the gut microbiome composition.
Studies have clearly indicated the positive link between the gut microbiome composition and the neuropathology of dementia. However, more investigations on the gut microflora is required to enable additional novel vision into the biology of dementia. This will facilitate in the development of novel therapeutic strategies for dementia. As stated in a review by Alkasir et al. (2017), the modulation of gut microbiota (by probiotics or other dietary intervention) or direct targeting of gut microbiota enzymes (by pharmacological inhibitors or activators) may be a growing area for pharmaceutical and functional food industries, with the goal of decreasing the widespread growth of adiposity, insulin resistance, Alzheimer’s disease, and other metabolic diseases.
Keywords:
Gut microbiome, Dysbiosis, Dementia, Alzheimer’s disease, Neuro-inflammation, Probiotics, Prebiotics
References:
- Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein & cell. 2017 Feb 1;8(2):90-102.
- Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci. 2016. doi: 10.1007/s11427-016-5083-9.
- Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutrition reviews. 2016 Oct 1;74(10):624-34.
Dementia, as a comprehensive type of brain disease is known to upset an individual’s daily activities. The disease is characterized by the deteriorated memory, thinking ability, behavior and the capability to carry out everyday functions. Globally, ~47.5 million individuals are affected and the prevalence of dementia is increasing continuously. Dementia leads to disability and dependency amongst the aged people, and thus has a colossal physical, mental, societal and economic effect on families, caregivers, and the general public. The common form of dementia is the Alzheimer’s disease, which account to about 60-70% of the cases followed by other forms, such as Lewy body dementia, vascular dementia, frontotemporal dementia, and Parkinson’s disease with dementia.
Recent research advancements have deciphered the role of gut microbiome and its composition associated with the gut permeability and inflammation in dementia patients. Also, it is hypothesized that dysbiosis (an impaired microbiota) may augment the gut permeability, microbial translocation and trigger an inflammatory-immune response, which may perhaps encourage pathogenesis and progression of dementia. In this regard, a novel approach is suggested for the management of these health disorders, and as an adjuvant for psychiatric treatment of neurological disorders, including dementia and other interrelated diseases via modulating the microbiota composition (e.g., with the use of probiotics).
The expansion of advanced high-throughput analytical techniques including next generation sequencing has disclosed the possible role of the commensal microbial population, particularly the gut microbiome in several human diseases. Lately, the theory of the gut brain-axis has been well-established. Accordingly, a communication exists between the gut and brain site to control and mediate the process of many biological pathways involving the autonomic and enteric nervous system, the neuroendocrine system and the immune system. However, any deviations in such communication due to dysbiosis may involve in disease development. For example, the enteric microbiota i.e., Bifidobacterium and Escherichia, which are considered as both commensal and pathogenic microbes are identified to have interactions between the brain and gut axis.
The gut microbiota supports numerous daily activities of the brain, comprising the regulation of the hypothalamic-pituitary-adrenal (HPA) axis activation state. The HPA axis activation releases cortisol, which in turn manage the triggering of microglia of the brain, and influence to release cytokines. This action rule the functions of the central nervous system through various communications, including vagal nerve and adrenergic nerve activation in addition to the secretion of many molecular candidates (endocrine hormones, neuropeptides, neurotransmitters, and immunomodulators). The stress hormones of hosts, for example noradrenaline might affect signalling between bacteria or their activities in the gut and lead to changes in the microbial diversity and overall functions of the gut microbiota. Nevertheless, these gut bacteria synthesize and release many neurotransmitters and neuromodulators, and induce the synthesis as well as the release of neuropeptides from enteroendocrine cells. For instance: the species of Bifidobacterium and Lactobacillus produce short-chain fatty acids; spore-forming bacteria produce 5-HT; Bacillus, Escherichia and Saccharomyces spp. secrete norepinephrine; Bacillus and Lactobacillus spp. produce dopamine and acetylcholine, respectively. In specific, recent findings have suggested that microglia maturation and function is governed by the short-chain fatty acids produced by the gut microbiota, and hence highlighted on the possible usefulness of the gut microbiome as an impending diagnostic target in dementia and its cure.
The gut microbiota regulates neuro-inflammations and the HPA axis activity, and might lead to Alzheimer’s disease. The neuropathological features of Alzheimer’s disease are the two pathologic protein aggregates, namely amyloid beta (Aβ) plaques and hyper-phosphorylated tangles of tau-protein, which cause neuro-inflammation, mainly facilitated by the innate immune system. This process mainly includes microglia cells that represent the resident macrophages of the brain. Though microglia cells can remove extracellular Aβ aggregates, in later phases of the disease, cells remain in a dystrophic state and fail to exert their positive actions. In addition, the inflammation and the pathogens (e.g., bacteria: Salmonella enterica; fungi: Candida albicans; viruses: herpes simplex virus; and parasites: Toxoplasma gondii) interactions can also lead to the development of Alzheimer’s disease. Thus, a strong gut microbiota may pay a significant role in preventing general infections by restricting pathogen growth, improving the microbial barriers, and enhancing the immune response. The representation of a few key factors in the Alzheimer’s disease pathogenesis is given in the figure-1.
Figure -1: Schematic of some key players in the pathogenesis of AD. The gut microbiota regulation of neuro-inflammation and the hypothalamic–pituitary–adrenal (HPA) axis activity and may lead to AD. The bacterial products that gain access to the brain through the bloodstream and the area postrema, via cytokine release from mucosal immune cells, through the release of gut hormones such as 5-HT from EEC cells, or via afferent neural pathways, including the vagal nerve. NP: Neuropeptide; NT: Neurotransmitter; 5-HT: 5-hydroxytryptamine; DC: Dendritic cell; EEC: Enteroendocrine cell; Aβ: amyloid beta protein; AD: Alzheimer’s disease.
(Adapted from Alkasir et al. 2017; doi: 10.1007/s13238-016-0338-6).
In the course of ageing, the gut microbiome composition undergoes fluctuations. In aged people, decreased microbial diversity with a loss of helpful taxa and increased facultative pathogens have been observed. Further, aging is connected with inflammation, which is also represented as inflammaging, which is linked with the accelerated gut permeability, mucosal inflammation and bacterial translocation. As aging is one of the chief risk factors of dementia, it is expected that the gut-brain axis is disapprovingly involved in the development of dementia. Considering this, a precise diet and the surrounding environment play a vital role in shaping the good microbiome composition and help in controlling many metabolic disorders. Some of the animal investigations have suggested that dementia is associated with fluctuations in the gut microbiome composition.
Studies have clearly indicated the positive link between the gut microbiome composition and the neuropathology of dementia. However, more investigations on the gut microflora is required to enable additional novel vision into the biology of dementia. This will facilitate in the development of novel therapeutic strategies for dementia. As stated in a review by Alkasir et al. (2017), the modulation of gut microbiota (by probiotics or other dietary intervention) or direct targeting of gut microbiota enzymes (by pharmacological inhibitors or activators) may be a growing area for pharmaceutical and functional food industries, with the goal of decreasing the widespread growth of adiposity, insulin resistance, Alzheimer’s disease, and other metabolic diseases.
Keywords:
Gut microbiome, Dysbiosis, Dementia, Alzheimer’s disease, Neuro-inflammation, Probiotics, Prebiotics
References:
- Alkasir R, Li J, Li X, Jin M, Zhu B. Human gut microbiota: the links with dementia development. Protein & cell. 2017 Feb 1;8(2):90-102.
- Hu X, Wang T, Jin F. Alzheimer’s disease and gut microbiota. Sci China Life Sci. 2016. doi: 10.1007/s11427-016-5083-9.
- Pistollato F, Sumalla Cano S, Elio I, Masias Vergara M, Giampieri F, Battino M. Role of gut microbiota and nutrients in amyloid formation and pathogenesis of Alzheimer disease. Nutrition reviews. 2016 Oct 1;74(10):624-34.
The gut microbiome promotes immune homeostasis
The gut microbiome is defined as the group of microorganisms and their genes existing inside the human gut. Recently, it has arisen as a primary factor in various human health conditions and diseases. A symbiotic relationship between humans and microbes is prevailing ever since from the birth, and any perturbations in this relationship could be the basis for immunological dysregulation, which might lead to the onset of many health complications and diseases including inflammatory bowel disease, autoimmunity, rheumatoid arthritis, cancer and multiple sclerosis. Symbiotic bacteria of the human gut provide a good environment and several advantages, such as providing essential nutrients, metabolizing indigestible food compounds, defense against the opportunistic pathogen colonization, and even contribute in the development of the intestinal architecture.
The immune system is responsible to recognize, retort and acclimatize to numerous foreign substances/molecules and also self-molecules. Thus, immune responses significantly modulate the development of health and disease. The human gut placidly co-exist with vast and diverse beneficial microbiota, and our immune system has evolved with gut bacteria to protect against various infectious microbial pathogens. In specific, the gastrointestinal tract is the main site at which both symbiotic and pathogenic microbes interact with the host immune system. More recent evidences have suggested a beneficial partnership has evolved amongst the symbiotic gut bacteria and the host immune system. The molecular communications/exchanges between them are directly linked to the growth of immune responses, and sequentially, the immune system of the host shapes the configuration of the microbiota.
Research evidences have related the role of the gut microbiota in shaping our immune system responses during diseases, however, the question still remains as to which specific bacteria are responsible in the mediation of these helpful responses and, more importantly, how this is accomplished. Some inspiring examples of the gut microorganisms having a pivotal role in the prevention of inflammatory bowel diseases, and beneficial immune reactions they stimulate during the defense are highlighted below.
At the start of 1900s, Ilya Mechnikov proposed for the first time about the use of live microbes to sustain bowel health and prolong life. Presently, the word “probiotic” is used for describing dietetic microbes with the ability of offering health benefits to the host. Numerous bacterial species either individually or in combination have been reported to ameliorate the symptoms of inflammatory bowel diseases in humans and mouse models. Some of them include Bifidobacteria lactis, B. infantis, E. coli Nissle 1917, Lactobacillus rhamnosus GG, L. salivarius, L. fermentum, Bacteriodes fragilis, B. thetaiotaomicron, etc. Mainly, these probiotic strains decrease toxic microbial metabolic events, however, more recent evidences have demonstrated their capability to modulate gut immune responses. The probiotic strains have a common feature of controlling the inflammation. The probiotic bacteria act on different cell types including epithelial cells, T cells and dendritic cells. Further, evidences have suggested that these probiotic strains induce regulatory T cells, which is the central to regulating inflammation and diseases. For example, treating colitic mice with the VSL#3 (a mixture of 8 lactic acid bacteria probiotic strains) increased the production of interleukin (IL-10) and the percentage of TGFβ-expressing T cells. Likewise, in another model of pathogen-induced inflammation study, the treatment of mice with B. infantis significantly down-regulated the intestinal inflammation and increased the number of CD4+CD25+ TReg cells. Moreover, the adaptive transmission of the CD4+CD25+ TReg-cells from mice served with B. infantis repressed inflammation-related activation of NF-κB (nuclear factor-κB) in the recipient mice. Interestingly, when the bone-marrow-derived dendritic cells incubated with L. rhamnosus were transferred into a colitic mice, a protection against inflammation and disease was observed. Further studies have shown that L. rhamnosus-treated dendritic cells can initiate TReg-cell activity. It has been shown that certain patients with Crohn's disease possess a reduced level of a noticeable gut bacteria, Faecalibacterium prausnitzii. As reviewed and explained by Round and Mazmanian (2009), this organism or its secreted substances are capable of inducing IL-10 expression (anti-inflammatory response), and ameliorate the induction of tumor necrosis factor-α (TNF-α) and bowel diseases, when administered orally to the experimental animals. This study further indicated that there exists a direct link between the reduced numbers of F. prausnitzii from the gut microbiota and the development of a bowel disease. This clearly indicates that symbiotic gut bacteria can intermediate in inflammatory bowel diseases and health. However, the explicit molecules/compounds secreted by these beneficial microbes of gut microbiota to guide in the immune responses still remains unclear. But, existing scientific data support the clue that symbiotic gut microbes actively interconnect with the host immune system to modulate anti-inflammatory processes.
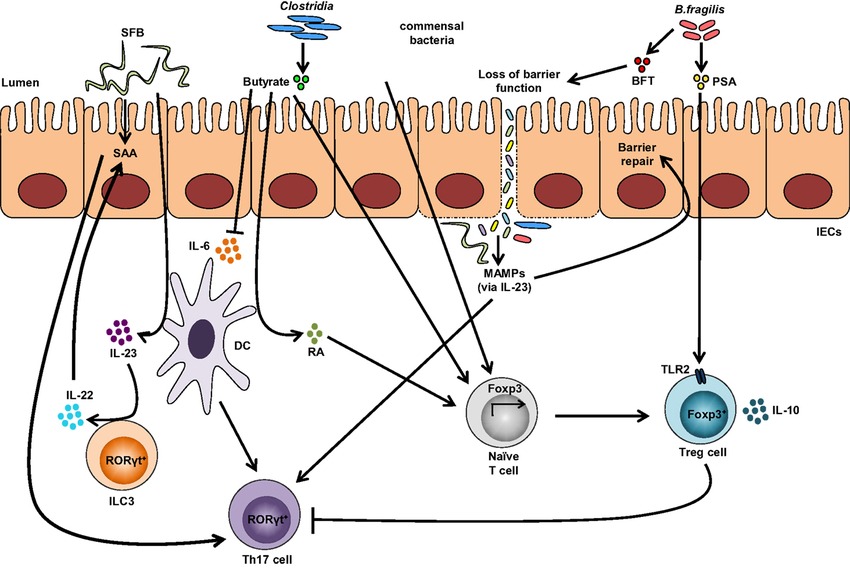
A single molecule, polysaccharide A (PSA) secreted by the human commensal bacterium (Bacteroides fragilis) is predicted to shape favorable immune responses (Figure 1). When the germ-free mice were colonized with B. fragilis or treated with the purified PSA, the cellular and physical development of the immune system was improved with the increase of differentiated splenic CD4+ T cells. Experimental evidences have suggested that PSA protects by decreasing the levels of the pro-inflammatory cytokines (TNFα, IL-17 and IL-23). Also, it inhibits epithelial hyperplasia and neutrophil infiltration to the gut associated with disease induction in these models (Round and Mazmanian 2009). Likewise, the gut microbiota on T-cells immune responses are depicted in figure 1.
Figure-1: Impact of the gut microbiota on T-cells immune responses. Colonization with segmented filamentous bacteria (SFB) occurs by intimate attachment to the intestinal epithelium and promotes the development of T-helper 17 (Th17) cells via intestinal epithelial cell (IEC)-derived cytokines, serum amyloid A (SAA), as well as antigen presentation by dendritic cells (DCs). Adhesion of SFB to IEC can potentially generate a circuit, wherein DC-derived IL-23 stimulates IL-22 production by type 3 innate lymphoid cells (ILC3), which in turn induces SAA from IEC and can lead to Th17 cell differentiation. Conversely, colonization of beneficial commensal bacteria induces de novo generation of Tregs and downregulates Th17 immune responses. Commensal bacteria, including most Clostridia species, produce short-chain fatty acids, i.e., butyrate, which participates in the de novo generation of T-regulatory cells by suppressing proinflammatory cytokines, by promoting RA production from DCs, and by inducing Foxp3 transcription. Among different Bacteroides fragilis strains, those expressing polysaccharide A (PSA) mediate the generation of Tregs via TLR2, while those secreting B. fragilis toxin (BFT) alter the function of IEC tight junctions. Upon disruption of barrier function, dissemination of microbial products, recognized by microbe-associated molecular patterns (MAMPs), occurs and activates the IL-23 pathway, resulting in subsequent barrier repair and stimulation of Th17 immune responses. (Adapted from Omenetti et al. 2015; https://doi.org/10.3389/fimmu.2015.00639).
Nevertheless, it can be predicted that some beneficial symbiont microbial species have evolved, and produce molecules that can prompt defensive intestinal immune reactions. The presently available treatments for inflammatory bowel diseases are either ineffectual in most patients or result in severe side effects. Therefore, understanding of the beneficial gut microbial species and their secreted compounds can aid in preventing or curing such diseases, and help in designing new and effective natural therapeutics against several inflammatory bowel diseases in near future.
References:
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009 May;9(5):313.
- Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Frontiers in immunology. 2015 Dec 17;6:639.
The gut microbiome is defined as the group of microorganisms and their genes existing inside the human gut. Recently, it has arisen as a primary factor in various human health conditions and diseases. A symbiotic relationship between humans and microbes is prevailing ever since from the birth, and any perturbations in this relationship could be the basis for immunological dysregulation, which might lead to the onset of many health complications and diseases including inflammatory bowel disease, autoimmunity, rheumatoid arthritis, cancer and multiple sclerosis. Symbiotic bacteria of the human gut provide a good environment and several advantages, such as providing essential nutrients, metabolizing indigestible food compounds, defense against the opportunistic pathogen colonization, and even contribute in the development of the intestinal architecture.
The immune system is responsible to recognize, retort and acclimatize to numerous foreign substances/molecules and also self-molecules. Thus, immune responses significantly modulate the development of health and disease. The human gut placidly co-exist with vast and diverse beneficial microbiota, and our immune system has evolved with gut bacteria to protect against various infectious microbial pathogens. In specific, the gastrointestinal tract is the main site at which both symbiotic and pathogenic microbes interact with the host immune system. More recent evidences have suggested a beneficial partnership has evolved amongst the symbiotic gut bacteria and the host immune system. The molecular communications/exchanges between them are directly linked to the growth of immune responses, and sequentially, the immune system of the host shapes the configuration of the microbiota.
Research evidences have related the role of the gut microbiota in shaping our immune system responses during diseases, however, the question still remains as to which specific bacteria are responsible in the mediation of these helpful responses and, more importantly, how this is accomplished. Some inspiring examples of the gut microorganisms having a pivotal role in the prevention of inflammatory bowel diseases, and beneficial immune reactions they stimulate during the defense are highlighted below.
At the start of 1900s, Ilya Mechnikov proposed for the first time about the use of live microbes to sustain bowel health and prolong life. Presently, the word “probiotic” is used for describing dietetic microbes with the ability of offering health benefits to the host. Numerous bacterial species either individually or in combination have been reported to ameliorate the symptoms of inflammatory bowel diseases in humans and mouse models. Some of them include Bifidobacteria lactis, B. infantis, E. coli Nissle 1917, Lactobacillus rhamnosus GG, L. salivarius, L. fermentum, Bacteriodes fragilis, B. thetaiotaomicron, etc. Mainly, these probiotic strains decrease toxic microbial metabolic events, however, more recent evidences have demonstrated their capability to modulate gut immune responses. The probiotic strains have a common feature of controlling the inflammation. The probiotic bacteria act on different cell types including epithelial cells, T cells and dendritic cells. Further, evidences have suggested that these probiotic strains induce regulatory T cells, which is the central to regulating inflammation and diseases. For example, treating colitic mice with the VSL#3 (a mixture of 8 lactic acid bacteria probiotic strains) increased the production of interleukin (IL-10) and the percentage of TGFβ-expressing T cells. Likewise, in another model of pathogen-induced inflammation study, the treatment of mice with B. infantis significantly down-regulated the intestinal inflammation and increased the number of CD4+CD25+ TReg cells. Moreover, the adaptive transmission of the CD4+CD25+ TReg-cells from mice served with B. infantis repressed inflammation-related activation of NF-κB (nuclear factor-κB) in the recipient mice. Interestingly, when the bone-marrow-derived dendritic cells incubated with L. rhamnosus were transferred into a colitic mice, a protection against inflammation and disease was observed. Further studies have shown that L. rhamnosus-treated dendritic cells can initiate TReg-cell activity. It has been shown that certain patients with Crohn's disease possess a reduced level of a noticeable gut bacteria, Faecalibacterium prausnitzii. As reviewed and explained by Round and Mazmanian (2009), this organism or its secreted substances are capable of inducing IL-10 expression (anti-inflammatory response), and ameliorate the induction of tumor necrosis factor-α (TNF-α) and bowel diseases, when administered orally to the experimental animals. This study further indicated that there exists a direct link between the reduced numbers of F. prausnitzii from the gut microbiota and the development of a bowel disease. This clearly indicates that symbiotic gut bacteria can intermediate in inflammatory bowel diseases and health. However, the explicit molecules/compounds secreted by these beneficial microbes of gut microbiota to guide in the immune responses still remains unclear. But, existing scientific data support the clue that symbiotic gut microbes actively interconnect with the host immune system to modulate anti-inflammatory processes.
A single molecule, polysaccharide A (PSA) secreted by the human commensal bacterium (Bacteroides fragilis) is predicted to shape favorable immune responses (Figure 1). When the germ-free mice were colonized with B. fragilis or treated with the purified PSA, the cellular and physical development of the immune system was improved with the increase of differentiated splenic CD4+ T cells. Experimental evidences have suggested that PSA protects by decreasing the levels of the pro-inflammatory cytokines (TNFα, IL-17 and IL-23). Also, it inhibits epithelial hyperplasia and neutrophil infiltration to the gut associated with disease induction in these models (Round and Mazmanian 2009). Likewise, the gut microbiota on T-cells immune responses are depicted in figure 1.
Figure-1: Impact of the gut microbiota on T-cells immune responses. Colonization with segmented filamentous bacteria (SFB) occurs by intimate attachment to the intestinal epithelium and promotes the development of T-helper 17 (Th17) cells via intestinal epithelial cell (IEC)-derived cytokines, serum amyloid A (SAA), as well as antigen presentation by dendritic cells (DCs). Adhesion of SFB to IEC can potentially generate a circuit, wherein DC-derived IL-23 stimulates IL-22 production by type 3 innate lymphoid cells (ILC3), which in turn induces SAA from IEC and can lead to Th17 cell differentiation. Conversely, colonization of beneficial commensal bacteria induces de novo generation of Tregs and downregulates Th17 immune responses. Commensal bacteria, including most Clostridia species, produce short-chain fatty acids, i.e., butyrate, which participates in the de novo generation of T-regulatory cells by suppressing proinflammatory cytokines, by promoting RA production from DCs, and by inducing Foxp3 transcription. Among different Bacteroides fragilis strains, those expressing polysaccharide A (PSA) mediate the generation of Tregs via TLR2, while those secreting B. fragilis toxin (BFT) alter the function of IEC tight junctions. Upon disruption of barrier function, dissemination of microbial products, recognized by microbe-associated molecular patterns (MAMPs), occurs and activates the IL-23 pathway, resulting in subsequent barrier repair and stimulation of Th17 immune responses. (Adapted from Omenetti et al. 2015; https://doi.org/10.3389/fimmu.2015.00639).
Nevertheless, it can be predicted that some beneficial symbiont microbial species have evolved, and produce molecules that can prompt defensive intestinal immune reactions. The presently available treatments for inflammatory bowel diseases are either ineffectual in most patients or result in severe side effects. Therefore, understanding of the beneficial gut microbial species and their secreted compounds can aid in preventing or curing such diseases, and help in designing new and effective natural therapeutics against several inflammatory bowel diseases in near future.
References:
- Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews Immunology. 2009 May;9(5):313.
- Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Frontiers in immunology. 2015 Dec 17;6:639.
- « Page 2 of 2.