Protecting Space Travelers Health Through 'Genomics' Approaches
In recent times, both public and private sectors are ambitious in sending more individuals to space via commercial suborbital space vehicles for various commercial endeavors including mining. Spaceflight, possessing the most life-threatening conditions are very challenging for humans to overcome from their adverse effects. A recent trend of increased space flight participants has resulted in higher heterogeneity in the astronaut’s long-term missions encountered with the exposure of the high radiations beyond low Earth orbit. Thus, vastly varying risk factors in addition to the abnormal unfriendly spacecraft’s conditions and space environments makes the normal humans to acclimatize easily. It has been reported that about 20-25 % of the long-term (>4 months) astronauts sent by the International Space Station (ISS) were reported to have persistent ocular complications soon after they complete their missions (Smith et al. 2012; Zwart et al. 2012). Likewise, some preliminary evidences suggest that astronauts may have altered one carbon metabolic effects due to genetic factors, micronutrients intake and the space flight environment (Schmidt and Goodwin 2013). Thus, it is necessary to consider such factors that may affect individuals and to develop defensive or adaptable environments. In this regard, to help and safeguard the progressively increasing number of astronauts, space researchers, and space visitors, it is necessary to recognize individual risk profiles to counteract such risks individually.
At present, astronauts receive cutting-edge medical attention, however genotypic and molecular assessments are lacked, which are very necessary as a countermeasures. Till date, only short-term cultures have been carried out onboard the ISS. However, long-term studies essentially involving culture-independent methods, such as high-throughput sequencing analyses are needed to examine how biological systems adapt to the space environment (Karouia et al. 2017). Further, to support the crew members, the use of emerging field of personalized medicine is highly advisable.
Astronauts are treated with Earth-based drugs for treating health issues during space missions. However, recent studies have stated the potential adverse effects of drug metabolisms and reactions that are based on an individual’s genotype. A medication given without knowing the genetic makeup of an individual will lead to adverse health outcomes and altered astronaut performance in space. Hence, personalized drug treatments for astronauts are to be considered. For instance, the characterization of individual astronaut’s biotransformation ability could be based on individual cytochrome P450 (CYP450) profiles, i.e., CYP450 gene family is a major subset of drug-metabolizing enzymes (Wu 2011). Likewise, personalized drug regimens is also based on the ethnicity of an individual. As stated by Schmidt et al. (2013), “most western people are characterized by roughly 93 % normal (or efficient) metabolizers, 7 % poor metabolizers, and 1 % ultra-rapid metabolizers of CYP2D6. In contrast, only 1 % of Asians are considered poor metabolizers of CYP2D6. Roughly 20 % of Asians are poor metabolizers via CYP2C19, while only about 4 % of Caucasians are considered poor metabolizers via this isoform”. Therefore, applications of pharmacogenomics could be useful as a personalize drug prescription for improving the safety and efficacy of drugs used in space. Further, assessment of specific genes, proteins, and metabolites that change due to drug administration will help in understanding the effects of drugs in space conditions. Likewise, it has been reported that long-duration crewmembers experience in-flight and post flight visual problems. However, the underlying etiology for these deviations is unfamiliar at the present time. The NASA medical community doubts on the fact that microgravity and radiations induces changes in the human physiology and chromosome instability (Otto 2012; Mader et al. 2012; Gibson et al. 2012; Schmidt et al. 2013). In this regard, metabolomics study could be useful in understanding the reasons for this altered physiological changes during and after space life. Likewise, molecular reasons that influence on the one carbon derangement on bone in the crewmembers in space could be envisaged by omics approaches. In a recent report, a comprehensive Whole Genome Analysis of Differential Epigenetic Effects of Space Travel on Monozygotic Twins (Twins Study – Feinberg) studies suggested the chemical changes in DNA is due to different environmental conditions found in space. They measured DNA methylation and chromatin at a genome-wide level in biological samples obtained from the space traveler before, during, and after flight, as well as from his ground-based twin. The study results suggested that the individual, who spent long-time in space had the predictive prolonged life when compared to ground based individual. This suggested that space conditions significantly influence on the DNA mutations, mainly attributed to radiations. However, detailed assessment is highly warranted to better understand on these observations.
Overall, personalized medicine makes use of a person’s information about genes, proteins and metabolites in response to diet, lifestyle and surrounding environment for diagnosing and preventing/treating diseases. With regard to spaceflight, it can be proposed that an optimized and a defined personalized diet/nutrition, practices, and atmosphere can be achieved for a better safety and performance of a space traveler under space conditions, which is mainly based on considering his/her genes, transcripts, proteins, and metabolites (Schmidt and Goodwin 2013). This will not only allow one to understand the profiles of astronauts individually, but also help in developing countermeasures so as to increase the capabilities of an astronaut to enthusiastically participate and perform to his/her highest competent level. In this regard, advancements in ‘omics’ approaches, such as genomics, transcriptomics, proteomics, and metabolomics are very useful in revealing unique molecular differences existing amongst persons (Karouia et al. 2017). It is necessary to mention that under extreme conditions, including spaceflight, such differences can be higher. Hence, knowing distinct variances in each astronauts will benefit in setting up of personalized countermeasure preparations that suit the best for each astronauts. Overall, high-throughput omics approaches will certainly give information on;
- The risks of individual differences among the individuals under space conditions,
- Behavior, in particular metabolism and regulation (including development),
- Genetic adaptations,
- Genetic variants that may alter risk and efficacy profiles of therapeutic drugs deployed in space,
- Metabolic variations and their influence on health,
- Molecular variants that may alter individual risk profiles in the high radiation environment of space,
- Identification of new microbes in space, and allow us to overcome some challenging concerns during space missions in future.
Key Words: Spacetraveler, Spacelife, Spaceflight, Genomics, Health Care, Genetic Variation, ISRO, NASA, Spacestation, ISS, Leucine Rich Bio, Genomic Signature.
References:
- Karouia F, Peyvan K, Pohorille A. Toward biotechnology in space: High-throughput instruments for in situ biological research beyond Earth. Biotechnology advances. 2017 Nov 15;35(7):905-32.
- Otto, C. (2012). NASA evidence report: Risk of spaceflight-induced intracranial hypertension/vision alterations. Version 1.0, Jul 12.
- Schmidt MA, Goodwin TJ. Personalized medicine in human space flight: using Omics based analyses to develop individualized countermeasures that enhance astronaut safety and performance. Metabolomics. 2013 Dec 1;9(6):1134-56.
- Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz?Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long?duration spaceflight: evidence from biochemistry and densitometry. Journal of Bone and Mineral Research. 2012 Sep 1;27(9):1896-906.
- Zwart SR, Gibson CR, Mader TH, Ericson K, Ploutz-Snyder R, Heer M, Smith SM. Vision changes after spaceflight are related to alterations in folate–and vitamin B-12–dependent one-carbon metabolism. The Journal of nutrition. 2012 Feb 1;142(3):427-31.
- Wu AH. Drug metabolizing enzyme activities versus genetic variances for drug of clinical pharmacogenomic relevance. Clinical proteomics. 2011 Dec;8(1):12.
In recent times, both public and private sectors are ambitious in sending more individuals to space via commercial suborbital space vehicles for various commercial endeavors including mining. Spaceflight, possessing the most life-threatening conditions are very challenging for humans to overcome from their adverse effects. A recent trend of increased space flight participants has resulted in higher heterogeneity in the astronaut’s long-term missions encountered with the exposure of the high radiations beyond low Earth orbit. Thus, vastly varying risk factors in addition to the abnormal unfriendly spacecraft’s conditions and space environments makes the normal humans to acclimatize easily. It has been reported that about 20-25 % of the long-term (>4 months) astronauts sent by the International Space Station (ISS) were reported to have persistent ocular complications soon after they complete their missions (Smith et al. 2012; Zwart et al. 2012). Likewise, some preliminary evidences suggest that astronauts may have altered one carbon metabolic effects due to genetic factors, micronutrients intake and the space flight environment (Schmidt and Goodwin 2013). Thus, it is necessary to consider such factors that may affect individuals and to develop defensive or adaptable environments. In this regard, to help and safeguard the progressively increasing number of astronauts, space researchers, and space visitors, it is necessary to recognize individual risk profiles to counteract such risks individually.
At present, astronauts receive cutting-edge medical attention, however genotypic and molecular assessments are lacked, which are very necessary as a countermeasures. Till date, only short-term cultures have been carried out onboard the ISS. However, long-term studies essentially involving culture-independent methods, such as high-throughput sequencing analyses are needed to examine how biological systems adapt to the space environment (Karouia et al. 2017). Further, to support the crew members, the use of emerging field of personalized medicine is highly advisable.
Astronauts are treated with Earth-based drugs for treating health issues during space missions. However, recent studies have stated the potential adverse effects of drug metabolisms and reactions that are based on an individual’s genotype. A medication given without knowing the genetic makeup of an individual will lead to adverse health outcomes and altered astronaut performance in space. Hence, personalized drug treatments for astronauts are to be considered. For instance, the characterization of individual astronaut’s biotransformation ability could be based on individual cytochrome P450 (CYP450) profiles, i.e., CYP450 gene family is a major subset of drug-metabolizing enzymes (Wu 2011). Likewise, personalized drug regimens is also based on the ethnicity of an individual. As stated by Schmidt et al. (2013), “most western people are characterized by roughly 93 % normal (or efficient) metabolizers, 7 % poor metabolizers, and 1 % ultra-rapid metabolizers of CYP2D6. In contrast, only 1 % of Asians are considered poor metabolizers of CYP2D6. Roughly 20 % of Asians are poor metabolizers via CYP2C19, while only about 4 % of Caucasians are considered poor metabolizers via this isoform”. Therefore, applications of pharmacogenomics could be useful as a personalize drug prescription for improving the safety and efficacy of drugs used in space. Further, assessment of specific genes, proteins, and metabolites that change due to drug administration will help in understanding the effects of drugs in space conditions. Likewise, it has been reported that long-duration crewmembers experience in-flight and post flight visual problems. However, the underlying etiology for these deviations is unfamiliar at the present time. The NASA medical community doubts on the fact that microgravity and radiations induces changes in the human physiology and chromosome instability (Otto 2012; Mader et al. 2012; Gibson et al. 2012; Schmidt et al. 2013). In this regard, metabolomics study could be useful in understanding the reasons for this altered physiological changes during and after space life. Likewise, molecular reasons that influence on the one carbon derangement on bone in the crewmembers in space could be envisaged by omics approaches. In a recent report, a comprehensive Whole Genome Analysis of Differential Epigenetic Effects of Space Travel on Monozygotic Twins (Twins Study – Feinberg) studies suggested the chemical changes in DNA is due to different environmental conditions found in space. They measured DNA methylation and chromatin at a genome-wide level in biological samples obtained from the space traveler before, during, and after flight, as well as from his ground-based twin. The study results suggested that the individual, who spent long-time in space had the predictive prolonged life when compared to ground based individual. This suggested that space conditions significantly influence on the DNA mutations, mainly attributed to radiations. However, detailed assessment is highly warranted to better understand on these observations.
Overall, personalized medicine makes use of a person’s information about genes, proteins and metabolites in response to diet, lifestyle and surrounding environment for diagnosing and preventing/treating diseases. With regard to spaceflight, it can be proposed that an optimized and a defined personalized diet/nutrition, practices, and atmosphere can be achieved for a better safety and performance of a space traveler under space conditions, which is mainly based on considering his/her genes, transcripts, proteins, and metabolites (Schmidt and Goodwin 2013). This will not only allow one to understand the profiles of astronauts individually, but also help in developing countermeasures so as to increase the capabilities of an astronaut to enthusiastically participate and perform to his/her highest competent level. In this regard, advancements in ‘omics’ approaches, such as genomics, transcriptomics, proteomics, and metabolomics are very useful in revealing unique molecular differences existing amongst persons (Karouia et al. 2017). It is necessary to mention that under extreme conditions, including spaceflight, such differences can be higher. Hence, knowing distinct variances in each astronauts will benefit in setting up of personalized countermeasure preparations that suit the best for each astronauts. Overall, high-throughput omics approaches will certainly give information on;
- The risks of individual differences among the individuals under space conditions,
- Behavior, in particular metabolism and regulation (including development),
- Genetic adaptations,
- Genetic variants that may alter risk and efficacy profiles of therapeutic drugs deployed in space,
- Metabolic variations and their influence on health,
- Molecular variants that may alter individual risk profiles in the high radiation environment of space,
- Identification of new microbes in space, and allow us to overcome some challenging concerns during space missions in future.
Key Words: Spacetraveler, Spacelife, Spaceflight, Genomics, Health Care, Genetic Variation, ISRO, NASA, Spacestation, ISS, Leucine Rich Bio, Genomic Signature.
References:
- Karouia F, Peyvan K, Pohorille A. Toward biotechnology in space: High-throughput instruments for in situ biological research beyond Earth. Biotechnology advances. 2017 Nov 15;35(7):905-32.
- Otto, C. (2012). NASA evidence report: Risk of spaceflight-induced intracranial hypertension/vision alterations. Version 1.0, Jul 12.
- Schmidt MA, Goodwin TJ. Personalized medicine in human space flight: using Omics based analyses to develop individualized countermeasures that enhance astronaut safety and performance. Metabolomics. 2013 Dec 1;9(6):1134-56.
- Smith SM, Heer MA, Shackelford LC, Sibonga JD, Ploutz?Snyder L, Zwart SR. Benefits for bone from resistance exercise and nutrition in long?duration spaceflight: evidence from biochemistry and densitometry. Journal of Bone and Mineral Research. 2012 Sep 1;27(9):1896-906.
- Zwart SR, Gibson CR, Mader TH, Ericson K, Ploutz-Snyder R, Heer M, Smith SM. Vision changes after spaceflight are related to alterations in folate–and vitamin B-12–dependent one-carbon metabolism. The Journal of nutrition. 2012 Feb 1;142(3):427-31.
- Wu AH. Drug metabolizing enzyme activities versus genetic variances for drug of clinical pharmacogenomic relevance. Clinical proteomics. 2011 Dec;8(1):12.
Identifying Evolutionary Diversity of Indian wolf through Next Generation Sequencing
Indian wolf (Canis lupus pallipes, synonym Canis indica), a subspecies of grey wolf (Canis lupus) is known to inhabit areas ranging from Southwest Asia to the Indian Subcontinent. These animals are intermediate in size between Arabian and Himalayan (Tibetan) wolf and possess no luxuriant winter coat found in other wolves residing in cold climate (1). However, they have short but less dense coat than other species of wolves, which is very fitting to their needs for survival under warm locations. In India, they dwell in habitats such as scrub-lands, thorn forests, arid and semi-arid grassland of Uttar Pradesh, Gujarat, Maharashtra and Karnataka (2). They survive by feeding on small animals, including rodents, raccoons and rabbits. Usually, they hunt during evening or night time. Indian wolf is also known to kill livestock and attack humans, hence, has become an enemy of the people in those regions. This behavior is mainly due to their lack of food in their natural environment as explained by experts.
In recent years, their population has significantly reduced and now they are considered as endangered. At present, only about 3,000 Indian wolves are believed to be present in the wild. Thus, they are protected by keeping in captivity at few locations such as the Jai Samand Sanctuary in Rajasthan. Because of their bad reputation and deprived monetary area where they live, conserving these animals is a major challenge. As most Indian wolves exist outside of protected Areas, and due to reduced natural prey species, they are forced to feed on livestock. This has resulted in conflict with local population leading to their reciprocal killings (3). Although they are protected due to being endangered, hunting still continues even today. With only a limited population left, it is obligatory to conserve Indian wolf species and to facilitate their breeding to increase their numbers.
Indian wolf (Canis lupus pallipes, synonym Canis indica)
They are suspected to have originated from the main ancestors of domestic dogs (4). Also, it is guessed that their challenging behavior with society would have led to their domestication as present day dogs and possibly towards their extinction. Due to the reddish to light brown color of the body, Indian wolf is sometimes confused as a fox when observed in the wild. However, some people erroneously speculate that Indian wolves to be grouped into a separate species. These wolves are not morphologically distinct from other Indian pallipes, and they don’t behave differently from them either. Thus, a lot of confusion are there with respect to its origin, distribution and species identification.
Although there is extensive literature on the wolf species, their ancient origin hypothesis is still not understood and is being debated. Indian wolf is genetically unique from all other wolves worldwide (5,6). It is hypothesized that the Himalayan wolf and the Indian gray wolf harbor most of the genetic diversity and are the most evolutionarily ancient lineages. They are being phylogenetically closer to jackal forms the basis for all other wolves and their divergence into different habitats (6).
Grey Wolf (Canis Lupus)
At present, only morphological and habitual behaviors are considered to trace their ancestral origin. However, studies based on the mitochondrial DNA sequences have indicated that the Indian gray wolf as the basal to all other extant Canis lupus haplotypes spaced out from the Himalayan wolf older-lineage (5,7). This basal position was further confirmed by comparing these sequences against worldwide wolf sequences (8,9,10). Likewise, a recent study was carried out to generate and compare the mitochondrial DNA sequences of ancient and modern wolves. The phylogenetic tree from the results indicated that both Indian gray wolf and the Himalayan wolf form the most basal (11). However, it is not possible to accept mtDNA alone as a basis for determining species status. Because, mitochondrial DNA sequences can only trace maternal lineages, and sometimes troubled with errors (12). In addition, most of the studies on Indian wolves involved wolves from India, which may not certainly refer to wolves with this old mtDNA sequence. Mitochondrial DNA is just a part of the genome, and what analyses of it actually prove are far narrower in scope than is often understood. While nuclear DNA studies are much harder to perform, but they are necessary before we start divining new species from those that have long been classified. Therefore, nuclear DNA investigations being done within Indian wolves is a must to interpret their ancestral lineage.
Genetic variation and its partitioning across populations provide information on the evolutionary history, demographic fluctuations, and population connectivity (13). More recently, genome-wide data are being analyzed to support an ancient or admixed origin hypothesis for the Holarctic gray wolf (Canis lupus) and the endemic coyote (Canis latrans) (14). They suggested the notion of unique ancestry as opposed to a hybrid origin. A rapidly advancing next-generation sequencing technologies has enabled the analysis of animal populations at the DNA sequence level (15). The next-generation sequencing is cost-effective and enables population-level studies of various organisms for the identification of their genotypic differences and phenotypic consequences (16). As a result, many large-scale population-based genome projects have been launched to analyze functional and evolutionary basis among animals such as tigers, dogs, forest elephants, chimpanzees, giant panda, etc. More recently, functional and evolutionary analysis of Korean bob-tailed native dog using whole-genome sequencing data has been successfully studied (16). However, attempts on analyzing the whole genome sequencing for Indian wolves is yet to be achieved. Till date, the genomic makeup or the present distribution of Indian wolves is not well characterized which is contributing problems to their ecological conservation.
Indian wolf (Canis lupus pallipes, synonym Canis indica), a subspecies of grey wolf (Canis lupus) is known to inhabit areas ranging from Southwest Asia to the Indian Subcontinent. These animals are intermediate in size between Arabian and Himalayan (Tibetan) wolf and possess no luxuriant winter coat found in other wolves residing in cold climate (1). However, they have short but less dense coat than other species of wolves, which is very fitting to their needs for survival under warm locations. In India, they dwell in habitats such as scrub-lands, thorn forests, arid and semi-arid grassland of Uttar Pradesh, Gujarat, Maharashtra and Karnataka (2). They survive by feeding on small animals, including rodents, raccoons and rabbits. Usually, they hunt during evening or night time. Indian wolf is also known to kill livestock and attack humans, hence, has become an enemy of the people in those regions. This behavior is mainly due to their lack of food in their natural environment as explained by experts.
In recent years, their population has significantly reduced and now they are considered as endangered. At present, only about 3,000 Indian wolves are believed to be present in the wild. Thus, they are protected by keeping in captivity at few locations such as the Jai Samand Sanctuary in Rajasthan. Because of their bad reputation and deprived monetary area where they live, conserving these animals is a major challenge. As most Indian wolves exist outside of protected Areas, and due to reduced natural prey species, they are forced to feed on livestock. This has resulted in conflict with local population leading to their reciprocal killings (3). Although they are protected due to being endangered, hunting still continues even today. With only a limited population left, it is obligatory to conserve Indian wolf species and to facilitate their breeding to increase their numbers.
Indian wolf (Canis lupus pallipes, synonym Canis indica)
They are suspected to have originated from the main ancestors of domestic dogs (4). Also, it is guessed that their challenging behavior with society would have led to their domestication as present day dogs and possibly towards their extinction. Due to the reddish to light brown color of the body, Indian wolf is sometimes confused as a fox when observed in the wild. However, some people erroneously speculate that Indian wolves to be grouped into a separate species. These wolves are not morphologically distinct from other Indian pallipes, and they don’t behave differently from them either. Thus, a lot of confusion are there with respect to its origin, distribution and species identification.
Although there is extensive literature on the wolf species, their ancient origin hypothesis is still not understood and is being debated. Indian wolf is genetically unique from all other wolves worldwide (5,6). It is hypothesized that the Himalayan wolf and the Indian gray wolf harbor most of the genetic diversity and are the most evolutionarily ancient lineages. They are being phylogenetically closer to jackal forms the basis for all other wolves and their divergence into different habitats (6).
Grey Wolf (Canis Lupus)
At present, only morphological and habitual behaviors are considered to trace their ancestral origin. However, studies based on the mitochondrial DNA sequences have indicated that the Indian gray wolf as the basal to all other extant Canis lupus haplotypes spaced out from the Himalayan wolf older-lineage (5,7). This basal position was further confirmed by comparing these sequences against worldwide wolf sequences (8,9,10). Likewise, a recent study was carried out to generate and compare the mitochondrial DNA sequences of ancient and modern wolves. The phylogenetic tree from the results indicated that both Indian gray wolf and the Himalayan wolf form the most basal (11). However, it is not possible to accept mtDNA alone as a basis for determining species status. Because, mitochondrial DNA sequences can only trace maternal lineages, and sometimes troubled with errors (12). In addition, most of the studies on Indian wolves involved wolves from India, which may not certainly refer to wolves with this old mtDNA sequence. Mitochondrial DNA is just a part of the genome, and what analyses of it actually prove are far narrower in scope than is often understood. While nuclear DNA studies are much harder to perform, but they are necessary before we start divining new species from those that have long been classified. Therefore, nuclear DNA investigations being done within Indian wolves is a must to interpret their ancestral lineage.
Genetic variation and its partitioning across populations provide information on the evolutionary history, demographic fluctuations, and population connectivity (13). More recently, genome-wide data are being analyzed to support an ancient or admixed origin hypothesis for the Holarctic gray wolf (Canis lupus) and the endemic coyote (Canis latrans) (14). They suggested the notion of unique ancestry as opposed to a hybrid origin. A rapidly advancing next-generation sequencing technologies has enabled the analysis of animal populations at the DNA sequence level (15). The next-generation sequencing is cost-effective and enables population-level studies of various organisms for the identification of their genotypic differences and phenotypic consequences (16). As a result, many large-scale population-based genome projects have been launched to analyze functional and evolutionary basis among animals such as tigers, dogs, forest elephants, chimpanzees, giant panda, etc. More recently, functional and evolutionary analysis of Korean bob-tailed native dog using whole-genome sequencing data has been successfully studied (16). However, attempts on analyzing the whole genome sequencing for Indian wolves is yet to be achieved. Till date, the genomic makeup or the present distribution of Indian wolves is not well characterized which is contributing problems to their ecological conservation.
Studies Suggest the Relationship Between the Gut Microbiome and Allergic Diseases
The past few years have witnessed the increased cases of patients suffering from allergies and inflammatory diseases. Scientific explorations have suggested the existence of the relationship between the gut microbiome and allergic diseases. The gut microbiome produces both beneficial and damaging metabolites from the diet. Along with bacterial components, these metabolites modulate the host immunity, and significant influence on the occurrence of allergic illnesses.
The human gut harbors several trillions of commensal microorganisms, mainly the bacterial population with more than thousands of species. This collection of microbes is generally represented as the gut microbiome. Due to obvious reasons, culture-based analysis of microbiome diversity is replaced by the recent advanced high-throughput DNA sequencing approaches, such as the bacterial 16S ribosomal RNA sequencing and microbiomics. These approaches enable the correct documentation of commensal bacteria directly without requiring to culture. Such studies have evidenced that alteration in the gut microflora composition or declined diversity, also known as dysbiosis state may lead to the development of intestinal inflammations and subsequently cause allergic conditions. For example, in a study comprising patients having food allergies in the United States has revealed the occurrence of reduced microbial diversity in the gut, and found an increased number of bacterial species belonging to Bacteroidales, while Clostridiales were found in low numbers. Further, some gut bacteria produce useful metabolites using the host's diet, and they directly take part in the mediation of host’s immune responses. Researchers have highlighted the role of commensal gut bacteria and their metabolites in regulating host immune responses and allergic diseases.
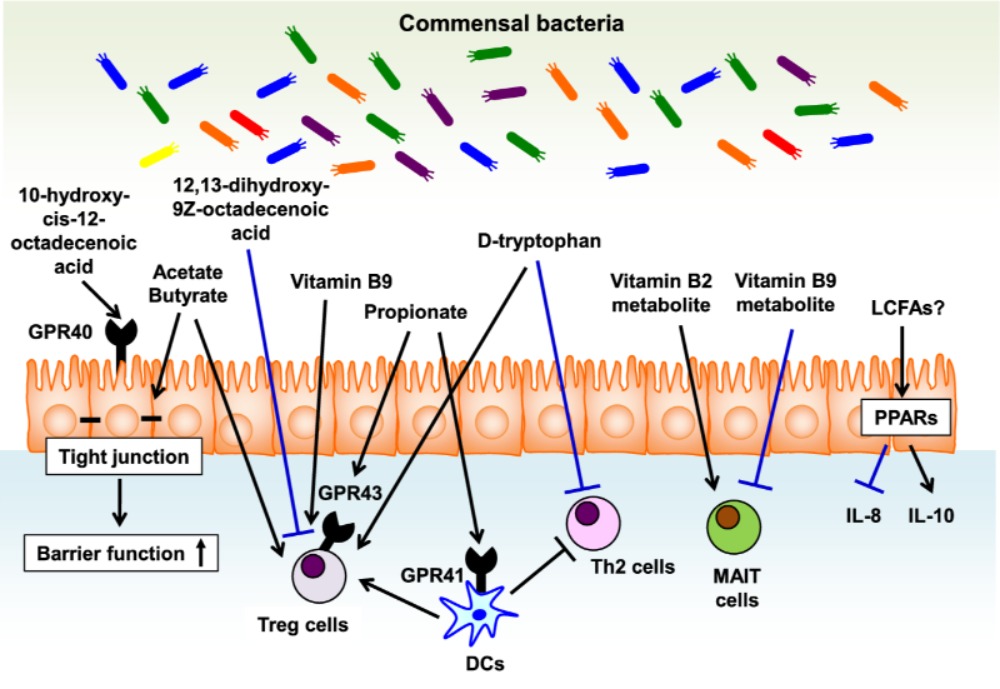
Long-chain fatty acids (LCFAs), such as ω3 and ω6 fatty acids function as energy sources and take part in the immune responses. However, mammals cannot produce LCFAs, and interestingly they are produced by the gut microbes using dietary components. Further, it is suggested that ω3 fatty acids have anti-inflammatory and anti-allergic properties (Miyata and Arita 2015). The gut commensal bacteria, mainly lactic acid bacteria (e.g., Lactobacillus plantarum) are reported to produce ω6 fatty acid-derived lipid metabolites (linoleic acids, oxo and hydroxy fatty acids). Likewise, 17,18-epoxy-eicosatetraenoic acid, an anti-allergic lipid metabolite is speculated to be produced in the colon via cytochrome P450 function. However, evidences have suggested the role of commensal bacteria in LCFAs metabolism. In a study, germ-free animals were shown to produce high levels of colonic lipid metabolites, such as 17-hydroxy docosahexaenoic acid, 14-hydroxy docosahexaenoic acid, resolvin D1, and protectin D1 when compared to conventional mice. Resolvin D1 is known to down-regulate the expression of interleukin-1β (IL-1β) during microbial infections. Further, IL-1β intensifies allergic reactions to cause asthma, atopic eczema, and dermatitis. Also, it is suggested that microbe-dependent inhibition of resolvin D1 production is linked to allergic inflammations (Hirata and Kunisawa 2017). Experimental evidences suggest that 10-hydroxy-cis-12-octadecenoic acid produced by Lactobacillus spp. may prevent the progress of food allergies, which is mediated by maintaining intestinal epithelial barrier function. Figure 1 illustrates the role of bacterial metabolites in the gut towards regulating immunity for the allergic diseases.
Figure-1: Bacterial metabolites in the gut regulate immune responses for the allergic diseases. Bacterial metabolites regulate versatile biologic and immunologic functions related to allergic diseases. Epithelial barrier function is enhanced by 10-hydroxy-cis-12-octadecenoic acid, a linoleic acid-derived metabolite, and SCFAs such acetate and butylate, fermentation products by some bacteria. In addition, SCFAs (e.g., acetate, butyrate, and propionate) as well as D-type tryptophan have a potential to enhance the induction of Treg cells. Vitamin B9 plays a key role in the maintenance of Treg cells and its metabolite prevents the activation of MAIT cells by competing with vitamin B2 metabolite, MAIT cell activating ligand, in their binding to MR1. PPAR ligands such as LCFAs decrease the inflammatory cytokines such as IL-8 and induce the production of anti-inflammatory cytokines such as IL-10. (Source: Hirata and Kunisawa 2017; https://doi.org/10.1016/j.alit.2017.06.008)
In a recent cohort study, the association amongst the gut microbiome, their lipids, and allergic diseases in humans was established. It was observed that the composition of the gut microbiota plays a significant role in the development of atopy and asthma in childhood. 16S rRNA sequencing analysis evidenced that high risk for the development of allergy in neonates is due to the reduced abundance of gut bacterial species, such as Bifidobacterium, Akkermansia and Faecalibacterium and increased abundance of fungi, such as Candida and Rhodotorula. Interestingly, the low-risk subjects were observed to have increased levels of anti-inflammatory lipid metabolites (docosapentaenoate and dihomo-γ-linolenate). On the other hand, high-risk groups were shown with enhanced levels of pro-inflammatory metabolites, such as 12,13-dihydroxy-9Z-octadecenoic acid, stigmasterol and sitosterol, and 8-hydoxyoctanoate (Fujimura et al. 2016). LPSAs are reported to modulate immune reactions via the peroxisome proliferator-activated receptor (PPAR) family, i.e., the most common negative regulators for allergic conditions. As stated by Hirata and Kunisawa (2017), Bacteroides thetaiotaomicron stimulates PPAR-γ-dependent export of NF-κB from the nucleus, and subsequently it decreases NF-κB-dependent IL-8 production to curb the IL-8-intermediated infiltration of granulocytes in bronchial allergy.
Moreover, immune cells, including iNKT (invariant natural killer T) cells are activated by microbial glycolipids. For example, Sphingomonas spp. producing glycosphingolipids, Sphingomonas spp., and Borrelia burgdorferi producing glycodiacylglycerols, Helicobacter pylori producing cholesteryl-α-glucoside. Further, D-type amino acids (e.g., D-alanine, D-glutamic acid, D-asparagine, and D-prorine) secreted from the gut bacteria are believed to control host allergic reactions. Likewise, D-tryptophan from the probiotic bacteria, such as Lactobacillus rhamnosus GG, Lactobacillus casei W56 is shown to ameliorate allergic airway inflammation and hyperresponsiveness in the gut and lung. Similarly, microbial vitamins, including B-family members and K, serve as an important supplementary source of vitamins that are take part in the regulation of the immune responses and controlling allergic diseases. Likewise, Short-chain fatty acids, such as propionate (produced from Bacteroidetes and some Firmicutes), acetate (produced from many genera of gut microflora, including Bifidobacterium spp.), butyrate (produced from Clostridium cluster XIVa species and Bacteroides thetaiotaomicron) exert diverse anti-allergic properties.
Overall, commensal gut bacteria produce many useful metabolites that are involved in regulating allergic responses mediated via various ways, such as the induction of Treg cells, up-regulation of IL-10 expression, suppression of the Th2-type phenotype and maintenance of the gut barrier function. Also, they are known to produce pro-inflammatory metabolites. There are many factors that are involved in the regulation of immune response and allergic diseases. Hence, having a right balance between diet and the gut microbiome may certainly benefit in overcoming allergic diseases. However, more research is highly recommended in this regard to clearly understand about the mechanisms involved and to disclose various cross-talk between commensal microbes in the gut and host immune system.
Keywords: The gut microbes, metabolites, microbiome, short-chain fatty acids, vitamins, allergic diseases, immunity
References:
- Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, Wegienka G. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nature medicine. 2016 Oct;22(10):1187.
- Hirata SI, Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergology International. 2017;66(4):523-8.
- Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergology International. 2015;64(1):27-34.
The past few years have witnessed the increased cases of patients suffering from allergies and inflammatory diseases. Scientific explorations have suggested the existence of the relationship between the gut microbiome and allergic diseases. The gut microbiome produces both beneficial and damaging metabolites from the diet. Along with bacterial components, these metabolites modulate the host immunity, and significant influence on the occurrence of allergic illnesses.
The human gut harbors several trillions of commensal microorganisms, mainly the bacterial population with more than thousands of species. This collection of microbes is generally represented as the gut microbiome. Due to obvious reasons, culture-based analysis of microbiome diversity is replaced by the recent advanced high-throughput DNA sequencing approaches, such as the bacterial 16S ribosomal RNA sequencing and microbiomics. These approaches enable the correct documentation of commensal bacteria directly without requiring to culture. Such studies have evidenced that alteration in the gut microflora composition or declined diversity, also known as dysbiosis state may lead to the development of intestinal inflammations and subsequently cause allergic conditions. For example, in a study comprising patients having food allergies in the United States has revealed the occurrence of reduced microbial diversity in the gut, and found an increased number of bacterial species belonging to Bacteroidales, while Clostridiales were found in low numbers. Further, some gut bacteria produce useful metabolites using the host's diet, and they directly take part in the mediation of host’s immune responses. Researchers have highlighted the role of commensal gut bacteria and their metabolites in regulating host immune responses and allergic diseases.
Long-chain fatty acids (LCFAs), such as ω3 and ω6 fatty acids function as energy sources and take part in the immune responses. However, mammals cannot produce LCFAs, and interestingly they are produced by the gut microbes using dietary components. Further, it is suggested that ω3 fatty acids have anti-inflammatory and anti-allergic properties (Miyata and Arita 2015). The gut commensal bacteria, mainly lactic acid bacteria (e.g., Lactobacillus plantarum) are reported to produce ω6 fatty acid-derived lipid metabolites (linoleic acids, oxo and hydroxy fatty acids). Likewise, 17,18-epoxy-eicosatetraenoic acid, an anti-allergic lipid metabolite is speculated to be produced in the colon via cytochrome P450 function. However, evidences have suggested the role of commensal bacteria in LCFAs metabolism. In a study, germ-free animals were shown to produce high levels of colonic lipid metabolites, such as 17-hydroxy docosahexaenoic acid, 14-hydroxy docosahexaenoic acid, resolvin D1, and protectin D1 when compared to conventional mice. Resolvin D1 is known to down-regulate the expression of interleukin-1β (IL-1β) during microbial infections. Further, IL-1β intensifies allergic reactions to cause asthma, atopic eczema, and dermatitis. Also, it is suggested that microbe-dependent inhibition of resolvin D1 production is linked to allergic inflammations (Hirata and Kunisawa 2017). Experimental evidences suggest that 10-hydroxy-cis-12-octadecenoic acid produced by Lactobacillus spp. may prevent the progress of food allergies, which is mediated by maintaining intestinal epithelial barrier function. Figure 1 illustrates the role of bacterial metabolites in the gut towards regulating immunity for the allergic diseases.
Figure-1: Bacterial metabolites in the gut regulate immune responses for the allergic diseases. Bacterial metabolites regulate versatile biologic and immunologic functions related to allergic diseases. Epithelial barrier function is enhanced by 10-hydroxy-cis-12-octadecenoic acid, a linoleic acid-derived metabolite, and SCFAs such acetate and butylate, fermentation products by some bacteria. In addition, SCFAs (e.g., acetate, butyrate, and propionate) as well as D-type tryptophan have a potential to enhance the induction of Treg cells. Vitamin B9 plays a key role in the maintenance of Treg cells and its metabolite prevents the activation of MAIT cells by competing with vitamin B2 metabolite, MAIT cell activating ligand, in their binding to MR1. PPAR ligands such as LCFAs decrease the inflammatory cytokines such as IL-8 and induce the production of anti-inflammatory cytokines such as IL-10. (Source: Hirata and Kunisawa 2017; https://doi.org/10.1016/j.alit.2017.06.008)
In a recent cohort study, the association amongst the gut microbiome, their lipids, and allergic diseases in humans was established. It was observed that the composition of the gut microbiota plays a significant role in the development of atopy and asthma in childhood. 16S rRNA sequencing analysis evidenced that high risk for the development of allergy in neonates is due to the reduced abundance of gut bacterial species, such as Bifidobacterium, Akkermansia and Faecalibacterium and increased abundance of fungi, such as Candida and Rhodotorula. Interestingly, the low-risk subjects were observed to have increased levels of anti-inflammatory lipid metabolites (docosapentaenoate and dihomo-γ-linolenate). On the other hand, high-risk groups were shown with enhanced levels of pro-inflammatory metabolites, such as 12,13-dihydroxy-9Z-octadecenoic acid, stigmasterol and sitosterol, and 8-hydoxyoctanoate (Fujimura et al. 2016). LPSAs are reported to modulate immune reactions via the peroxisome proliferator-activated receptor (PPAR) family, i.e., the most common negative regulators for allergic conditions. As stated by Hirata and Kunisawa (2017), Bacteroides thetaiotaomicron stimulates PPAR-γ-dependent export of NF-κB from the nucleus, and subsequently it decreases NF-κB-dependent IL-8 production to curb the IL-8-intermediated infiltration of granulocytes in bronchial allergy.
Moreover, immune cells, including iNKT (invariant natural killer T) cells are activated by microbial glycolipids. For example, Sphingomonas spp. producing glycosphingolipids, Sphingomonas spp., and Borrelia burgdorferi producing glycodiacylglycerols, Helicobacter pylori producing cholesteryl-α-glucoside. Further, D-type amino acids (e.g., D-alanine, D-glutamic acid, D-asparagine, and D-prorine) secreted from the gut bacteria are believed to control host allergic reactions. Likewise, D-tryptophan from the probiotic bacteria, such as Lactobacillus rhamnosus GG, Lactobacillus casei W56 is shown to ameliorate allergic airway inflammation and hyperresponsiveness in the gut and lung. Similarly, microbial vitamins, including B-family members and K, serve as an important supplementary source of vitamins that are take part in the regulation of the immune responses and controlling allergic diseases. Likewise, Short-chain fatty acids, such as propionate (produced from Bacteroidetes and some Firmicutes), acetate (produced from many genera of gut microflora, including Bifidobacterium spp.), butyrate (produced from Clostridium cluster XIVa species and Bacteroides thetaiotaomicron) exert diverse anti-allergic properties.
Overall, commensal gut bacteria produce many useful metabolites that are involved in regulating allergic responses mediated via various ways, such as the induction of Treg cells, up-regulation of IL-10 expression, suppression of the Th2-type phenotype and maintenance of the gut barrier function. Also, they are known to produce pro-inflammatory metabolites. There are many factors that are involved in the regulation of immune response and allergic diseases. Hence, having a right balance between diet and the gut microbiome may certainly benefit in overcoming allergic diseases. However, more research is highly recommended in this regard to clearly understand about the mechanisms involved and to disclose various cross-talk between commensal microbes in the gut and host immune system.
Keywords: The gut microbes, metabolites, microbiome, short-chain fatty acids, vitamins, allergic diseases, immunity
References:
- Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, Panzer AR, LaMere B, Rackaityte E, Lukacs NW, Wegienka G. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nature medicine. 2016 Oct;22(10):1187.
- Hirata SI, Kunisawa J. Gut microbiome, metabolome, and allergic diseases. Allergology International. 2017;66(4):523-8.
- Miyata J, Arita M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergology International. 2015;64(1):27-34.
- »